How To Effectively Manage Employee No Call No Show (With Example Policy)
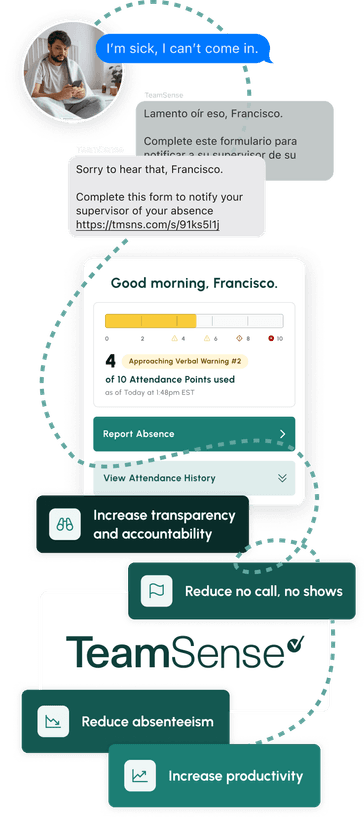
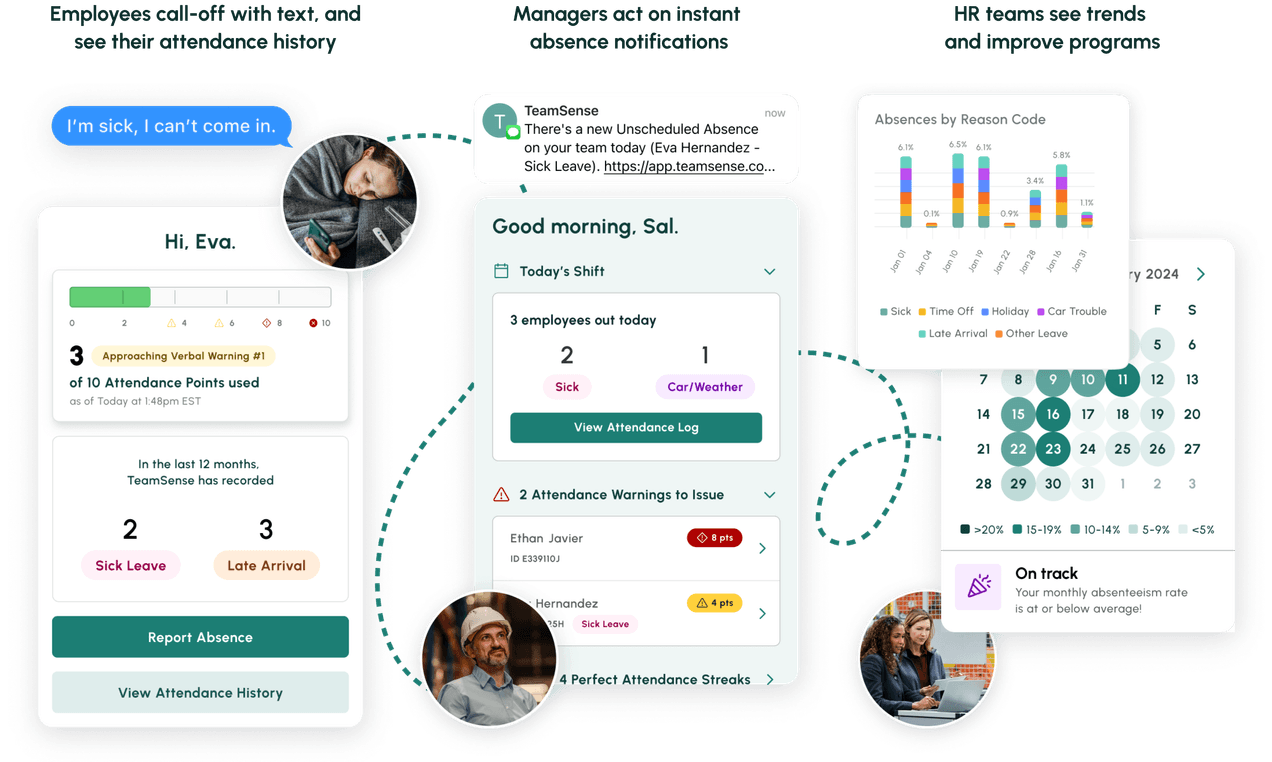
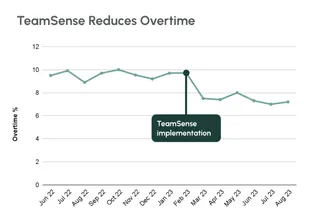
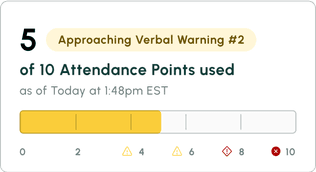
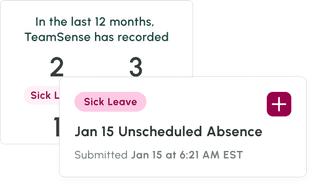
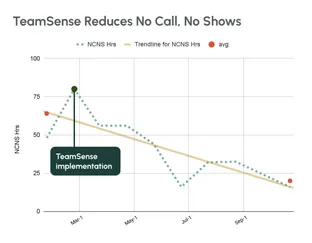
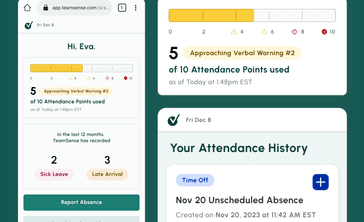
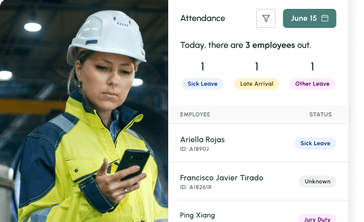
No call no shows are a problem in every industry, but especially so within the hourly workforce, where efficiency and productivity are at the heart of operations. Below we dive into how to handle no calls no shows, provide an example no call no show policy, and take a look at the impact of no call no show on employee morale and the company as a whole.